Abstract
Introduction: WhiMSICAL (Waldenström's Macroglobulinemia Study Involving CArt-wheeL) is the first global Waldenström's Macroglobulinemia (WM) registry capturing patient-derived data to complement scarce clinical trials data in this rare cancer (Tohidi-Esfahani et al, Am J Hematol 2021). The registry was interrogated to identify real-world first line treatment outcomes, quality of life (QoL) and coronavirus disease 2019 (COVID-19) data.
Methods: The registry captures data through www.cart-wheel.org, an online rare cancer database, utilizing a tailored questionnaire developed by clinician and patient investigators. WM patients complete consent online, then enter symptom, pathology, treatment, QoL (EORTC QLQ-C30) and COVID-19 data, and can return to update their data on an ongoing basis. Recruitment is driven by social media messaging by the International Waldenström's Macroglobulinemia Foundation investigators. Time to next treatment (TTNT) was assessed from start of first therapy to start of second therapy. Patients without a documented second therapy were censored at the time of last edit to their account. COVID-19 questions included testing, disease severity, vaccination and impact on WM management.
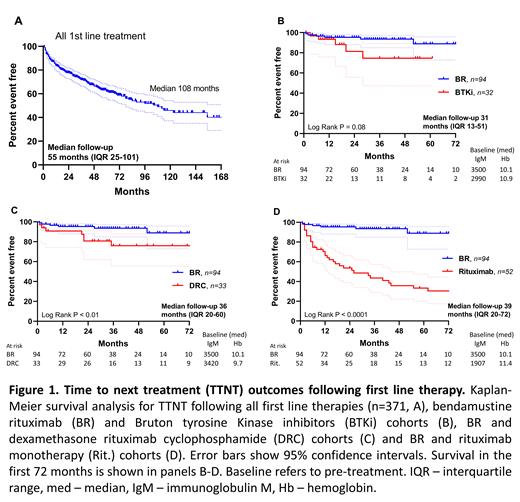
Results: As of July 2021, 558 patients from 20 countries have participated in the registry, predominantly from USA (50%), Australia (22%) and the UK (9%). Median age at diagnosis was 61 years (range 24-83) with male predominance (61%). 371 patients documented first-line therapies, with a total of 54 unique therapeutic combinations listed. The seven most common therapies were: bendamustine rituximab (BR, n=94), rituximab monotherapy (Rit., n=52), dexamethasone rituximab cyclophosphamide (DRC, n=33), ibrutinib (n=25), bortezomib dexamethasone rituximab (n=15), rituximab cyclophosphamide vincristine prednisolone (n=14) and chlorambucil (n=10). Comparison of TTNT was limited to the four most common first-line therapies: BR, Rit., DRC, with zanubrutinib (n=5) and ibrutinib plus rituximab (n=2) adding to the first line Bruton tyrosine Kinase inhibitor (BTKi) cohort (n=32). Median ages for the BR, BTKi, DRC and Rit. cohorts were 65, 66, 61 & 65 years, respectively. More patients in the BR cohort listed comorbidities (37%), with BTKi-treated patients reporting the least (19%). Pre-treatment disease burden (median IgM and hemoglobin) trended to being higher in the BR and DRC cohorts (figure 1B-D, IgM p=0.24, Hb p=0.27). At median follow up ranging from 31 to 39 months, BR had superior TTNT to DRC (median: not reached and 104 months, p=0.007, figure 1C) and Rit. (median 26 months, p < 0.0001, figure 1D), and trended to superiority compared to BTKi (median not reached, p=0.08, figure 1B). Median TTNT for the entire cohort (n=371) was 108 months (median follow up 55 months, figure 1A).
Assessment of QoL was conducted in all patients (any line of treatment) and compared between patients currently on BTKi therapy (n=64) and patients not exposed to BTKi and treated within the last 12 months (n=84). The expanded BTKi cohort reported better QoL, with mean EORTC QLQ-C30 global scale of 82 ± 14.4 compared to the BTKi-naïve cohort mean 73.4 ± 20.9, p=0.005. This was despite more prior lines of treatment (median 2 [IQR 1-4] compared to 1 [IQR 1-1]; p<0.0001).
324 (58%) patients responded to the COVID-19 questions. 144/324 (44%) had undergone testing for COVID-19, with 11 (8%) returning a positive result; none after vaccination. Median length of symptoms was seven days (range 2-30), with two hospitalized, one requiring intensive care. Both hospitalized patients were on second line ibrutinib. Of 211 responses regarding vaccination status, 15 (7%) were not vaccinated, eight due to availability, five due to personal choice and two due to clinician advice. Regarding impact of the pandemic on their WM management, 5% had treatment schedule disruption and 53% reported reduced face-to-face consultations.
Conclusion: The WhiMSICAL registry provides a scientifically robust and ethically approved portal for the patients' voice. The data highlight the real-world efficacy of combination chemoimmunotherapy, particularly first-line BR, and suggest a better QoL with BTKi than other therapies. As this global data platform grows, the breadth of data allows for new insights into WM with patient reported outcomes advancing knowledge and facilitating treatment decisions for clinicians and patients.
D'Sa: Sanofi: Honoraria; BeiGene: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding. Kersten: Roche: Consultancy, Honoraria, Other: Travel support, Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Other: Travel support; Novartis: Consultancy, Honoraria, Other: Travel support; BMS/Celgene: Consultancy, Honoraria; Takeda: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding; Celgene: Research Funding. Thomas: Acerta Pharma: Research Funding; Ascentage Pharma: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; X4 Pharma: Research Funding; Genentech: Research Funding. Palomba: Ceramedix: Honoraria; Rheos: Honoraria; Nektar: Honoraria; Priothera: Honoraria; Lygenesis: Honoraria; WindMIL: Honoraria; Wolters Kluwer: Patents & Royalties; Juno: Patents & Royalties; BeiGene: Consultancy; Kite: Consultancy; Magenta: Honoraria; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; PCYC: Consultancy; Notch: Honoraria, Other: Stock; Novartis: Consultancy; Pluto: Honoraria. Olszewski: Acrotech Pharma: Research Funding; Celldex Therapeutics: Research Funding; TG Therapeutics: Research Funding; PrecisionBio: Research Funding; Genentech, Inc.: Research Funding; Genmab: Research Funding. Trotman: PCYC: Research Funding; roche: Research Funding; BMS: Research Funding; TAKEDA: Research Funding; JANSSEN: Research Funding; beigene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal